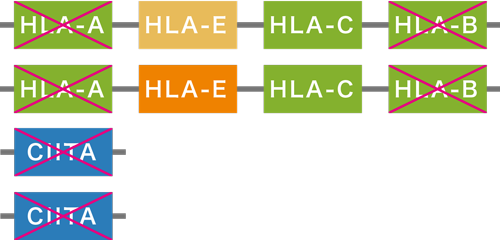
iPS Cell Stock Project HLA-genome-edited iPS cells
HLA-genome-edited iPS cells
HLA-genome-edited iPS cells list
Clinical-grade cells
| HLA-genome-edited iPS cells | For profit | Non-profit institution |
|---|---|---|
| Clinical-grade cells | 200,000 yen/vial | free |
✓It is provided free of charge to non-profit organizations.
✓Approval from the iPS Cell Stock Committee is required before cells can be provided.
✓Even when you apply for research purpose (non-clinical), a clinical-grade cell line is provided.Under the research condition, do not use in humans.
✓International Shipping: Receiving Organization, whether profit or non-profit, is required to arrange for a shipping and transportation from CiRA Foundation to its research location by its cost. CiRA Foundation will not responsible for any of loss and damages of iPSC stock during the shipment or the transportation.
| Donor ID | Gene Knockout | Clone ID |
|---|---|---|
| QHJI | ○ | QHJI14s04-AB II-KO-03 |
| QHJI | ○ | QHJI14s04-AB II-KO-11 |
| QHJI | ○ | QHJI14s04-AB II-KO-12 |
Since the clinical-grade cells with genome editing can also be used for research purpose, we generally recommend this clinical one for regenerative medicine.
It is also possible to provide the following research-grade cells with genome editing. However, please note that this research-grade cells are not same batch as the above clinical-grade cells, and the two lines are completely different in the manufacturing processes including the genome editing manipulation.
Research-grade cells
| HLA-genome-edited iPS cells | For profit | Non-profit institution |
|---|---|---|
| Research-grade cells | 100,000 yen/vial | free |
✓It is provided free of charge to non-profit organizations.
✓Approval from the iPS Cell Stock Committee is required before cells can be provided.
✓International Shipping: Receiving Organization, whether profit or non-profit, is required to arrange for a shipping and transportation from CiRA Foundation to its research location by its cost. CiRA Foundation will not responsible for any of loss and damages of iPSC stock during the shipment or the transportation.
| Donor ID | Gene Knockout | Clone ID |
|---|---|---|
| DRXT | × | Ff-XT28s 05-cont |
| ○ | Ff-XT28s05-ABo_To | |
| QHJI | ○ | Ff-I01s04-AB II-KO-16 |
| ○ | Ff-I01s04-AB II-KO-50 | |
| ○ | Ff-I01s04-AB II-KO-54 | |
| ○ | Ff-I14s04-AB II-KO-7 | |
| ○ | Ff-I14s04-AB II-KO-13 | |
| ○ | Ff-I14s04-AB II-KO-24 |
Related Information
- For Researchers and Companies
- Research Materials(Bioresources)
- iPS Cell Stock Project
- How to Use the Stock