Clinical Development
Allogeneic iPS cell-derived dopamine neural progenitor cells transplantation therapy for Parkinson’s disease

Sumitomo Pharma Co., Ltd.

In partnership with the Center for iPS Cell Research and Application (CiRA) at Kyoto University, Sumitomo Pharma is developing allogeneic iPS cell-derived dopaminergic neural progenitor cells. In February 2017, this project was designated for “SAKIGAKE Designation System” for regenerative medical products by the Ministry of Health, Labour and Welfare (MHLW).

Cell manufacturing
Kyoto University Hospital has conducted investigator-initiated clinical study since 2018. For the fourth and latter patients, we have provided cell products manufactured in our GMP manufacturing plant, SMaRT (Sumitomo Pharma Manufacturing Plant for Regenerative Medicine & Cell Therapy, located in Suita, Osaka). As announced by Kyoto University on January 11, 2022, seven patients have received transplantations. Now they are under two-year observation to evaluate safety and efficacy of the product.
Using the data obtained through these investigator-initiated clinical study, Sumitomo Pharma aims to launch as a regenerative medical product in Japan in FY2024. We have also scheduled a company initiated clinical study in the United States.
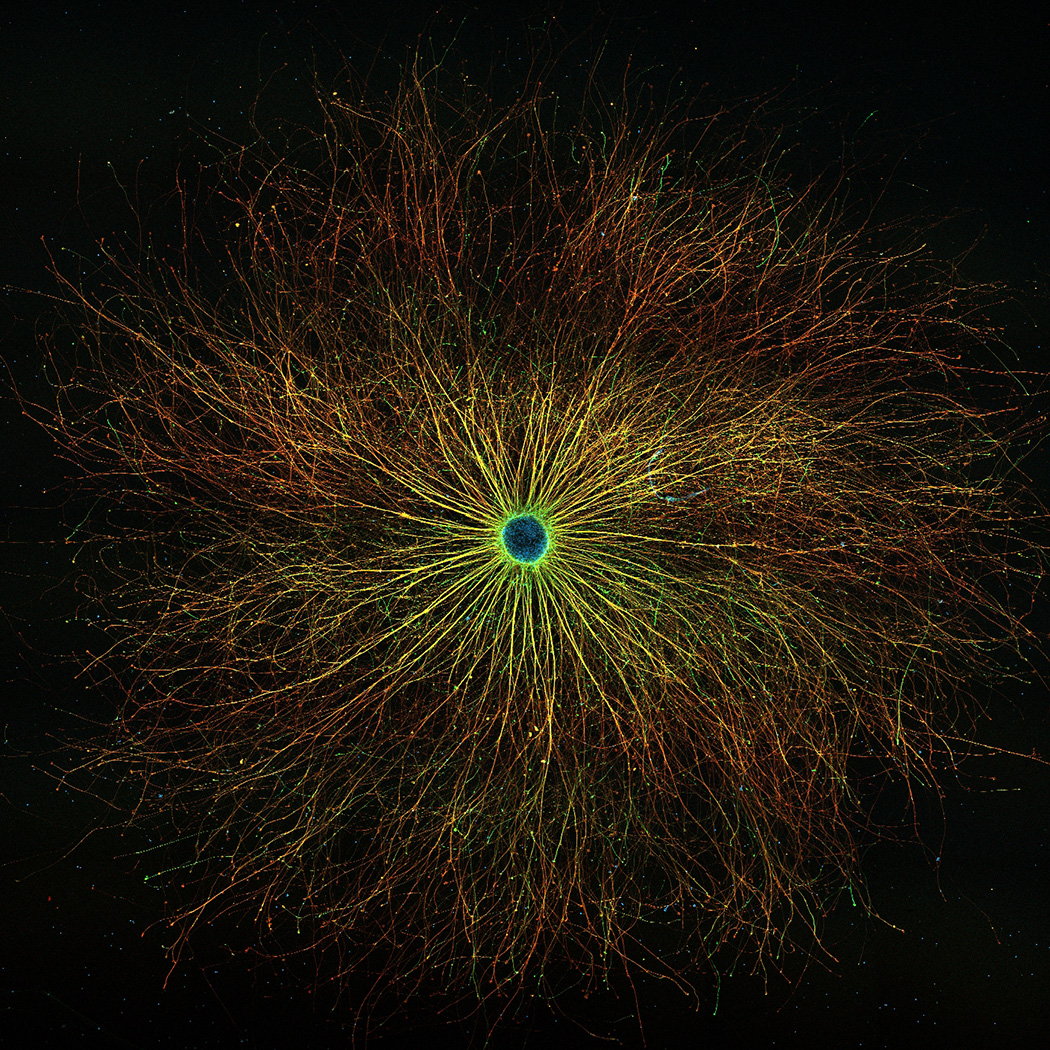
iPS cell-derived dopaminergic neural progenitor cells cultivated over a long period on a culture dish


