my iPS® Project

We are developing technologies to promote the clinical application of autologous induced pluripotent stem (iPS) cells.
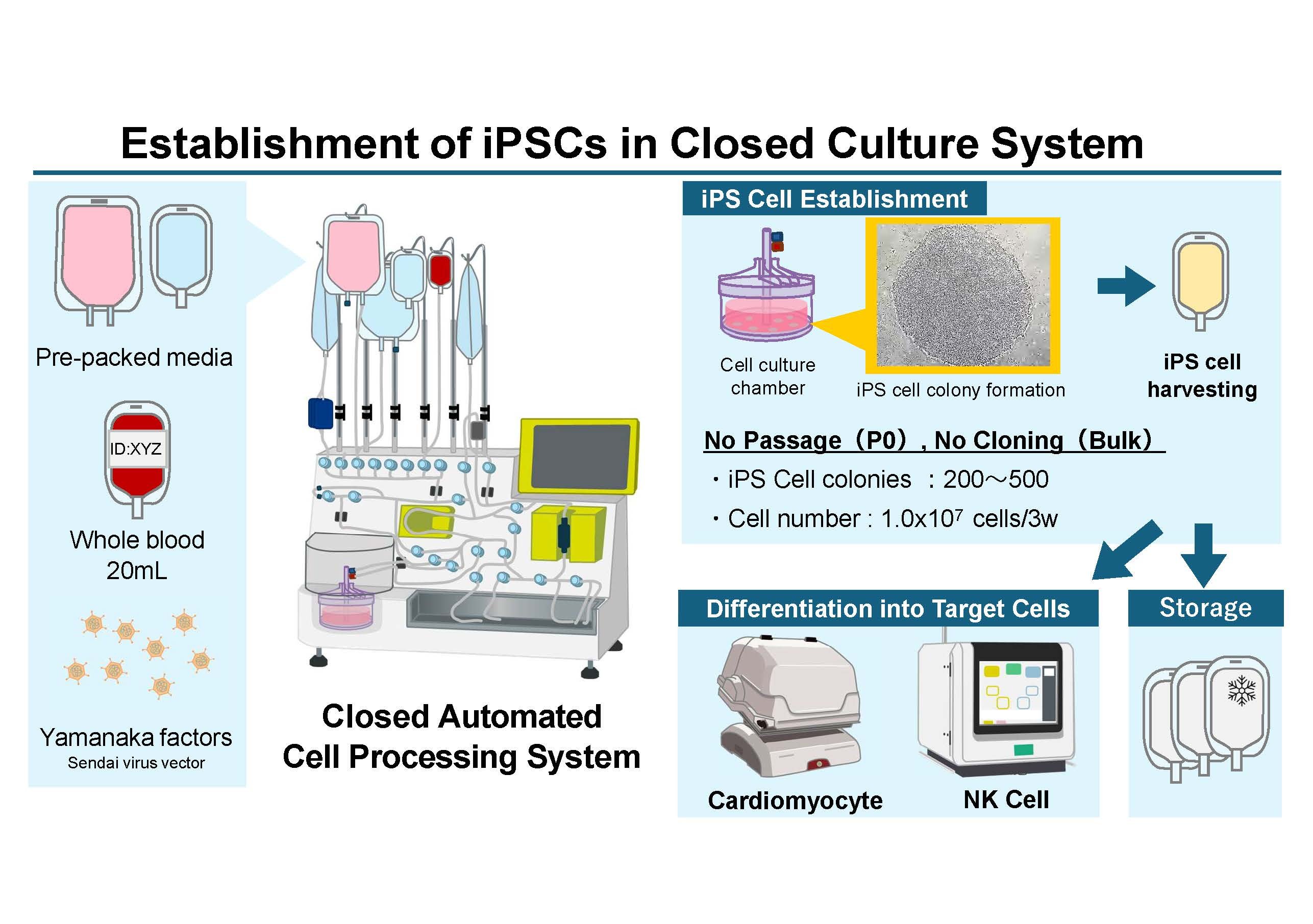
Currently, the iPS cells provided by the CiRA Foundation are manufactured manually by skilled operators in specialized facilities, and the production cost for a single manufacturing line amounts to tens of millions of yen.
Through the my iPS Project, we aim to significantly reduce the manufacturing cost of autologous iPS cells by developing closed, automated cell processing systems in collaboration with industry partners.
Overview
The my iPS Project aims to realize regenerative medicine using autologous iPS cells, which are generated from a patient’s own cells.
When regenerative therapies using iPS cells become widely available in the future, producing transplantable cells from a patient’s own iPS cells is expected to minimize the risk of immune rejection, as the cells are genetically identical to the recipient.
However, current iPS cell manufacturing relies on labor-intensive manual processes performed in specialized facilities, resulting in extremely high production costs—often reaching tens of millions of yen per manufacturing run.
The CiRA Foundation’s clinical-grade iPS cell stock (iPSC Stock), which consists of allogenic iPS cells and is also produced using these conventional processes, faces the same challenge in terms of per-batch manufacturing costs.
By contrast, allogeneic iPS cells offer an important advantage: because pre-manufactured, quality-assured iPS cells can be shared among multiple patients, the cost per-patient can be significantly reduced.
Nevertheless, depending on a patient’s individual characteristics or disease, there are cases in which generating transplantable cells from autologous iPS cells may be more suitable than using allogeneic iPS cells.
To address this challenge, we are working to automate the complex manufacturing processes that have traditionally relied on manual labor, with the goal of producing high-quality autologous iPS cells at significantly lower cost.
Ultimately, we aim to establish a system capable of supplying autologous iPS cells to research institutions and companies at a cost of approximately 1 million yen per patient.
Goals
Our ultimate goal is to expand regenerative medicine using autologous iPS cells into a form of treatment that anyone can access.
We aim to establish a system that enables the provision of iPS cells for individual patients at a cost of approximately 1 million yen to research institutions and companies, and to support the initiation of clinical trials by recipient institutions by the end of March 2029.
To achieve this, it is essential not only to reduce manufacturing costs but also to verify the safety and efficacy of cells produced through automated manufacturing.
As a first step, the CiRA Foundation will conduct non-clinical (safety) studies prior to human use, ensuring that the cells can be used with confidence in clinical trials conducted by collaborating medical institutions.
Challenges Toward Automated Manufacturing
We are advancing research and development to enable automated cell production using closed automated processing systems, which allow cells to be expanded while protecting them from contamination—replacing processes that were previously performed manually.
Our specific initiatives include:
- Development of systems that automate processes from iPS cell generation through differentiation into target cell types
- Establishment of three-dimensional (3D) culture methods optimized for automated systems
- Kit-based integration of reagents and materials compatible with automated devices
- Development of non-destructive quality assessment methods using image-based analysis
By realizing these technologies, we aim to enable the production of cells at substantially lower cost while maintaining high quality.
Yanai Facility for my iPS Cell Therapy

Monitoring Room

Manufacturing Room

Automated manufacture testing
Uehiro Laboratory for my iPS Cell Research

Working in the lab

Multiple devices installed in the lab

Research and development of automated manufacturing methods
For Companies and Researchers
At the CiRA Foundation, we are working on the development of closed culture systems and non-destructive quality assessment technologies. To reduce costs while ensuring safety and efficacy, we are seeking companies and research institutions willing to collaborate with us in tackling these challenges.
Research on the Generation of Specified Embryos (Animal–Human Chimeric Embryos)
In addition to efforts to reduce iPS cell manufacturing costs, the my iPS Project is also engaged in basic research aimed at realizing future medical applications that leverage the advantages of autologous iPS cells derived from a patient’s own cells.
Based on previous studies*¹, this research investigates how human iPS cells engraft and differentiate within pig embryos.
While this approach does not immediately enable the generation of transplantable organs, further optimization of experimental conditions may, in the future, enable the growth of patient-specific organs within animal hosts using autologous iPS cells.
At present, we are introducing human iPS cells into pig embryos and examining how these cells engraft and differentiate during development.
Our long-term vision is to realize a future in which organs generated from a patient’s own cells can be transplanted, offering new hope to patients who suffer while waiting for organ transplantation.
*¹ Previous research on organ generation using animal embryos
Human iPS cells were introduced into genetically modified pig embryos lacking kidney development (SIX1/SALL1 knockout) using the blastocyst complementation method.
Wang et al., Cell Stem Cell, 2023 「Generation of a humanized mesonephros in pigs from induced pluripotent stem cells
via embryo complementation」
To enhance cell survival, anti-apoptotic genes (MYCN, BCL2) were introduced into the iPS cells, and culture conditions were optimized.
As a result, at embryonic day 28, successful formation of mesonephros structures composed of 50–65% human-derived cells were achieved.
Wang et al., Cell Stem Cell, 2023:
“Generation of a humanized mesonephros in pigs from induced pluripotent stem cells via embryo complementation”
