HLA genome-edited iPS cells
HLA genome-edited iPS cells are designed to reduce the risk of immune rejections. We manufacture and provide genome-edited iPS cells knocked out for HLA-A, -B, and -CIITA.
About HLA genome-edited iPS cells
Advantages
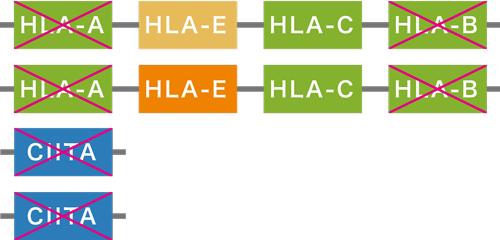
It has been estimated that only seven HLA genome-edited iPS cell lines could cover 95% of the Japanese population, and 12 cell lines could cover the entire world. An even smaller number of cell lines could serve most of the world's population. In addition, compared to cells which have had their HLA completely knocked out to reduce the risk of immune rejections, our cells are capable of avoiding both T and NK cell activity, because they retain HLA-C and HLA-E.
We generated HLA genome-edited iPS cells using CRISPR-Cas9 to knock out HLA-A, HLA-B, and the CIITA gene, which is important for HLA-Class II expression.
Future challenges
While the risk of immune rejection is reduced by HLA gene editing, it is not completely eliminated. Genome editing technologies for clinical application have never been tested in Japan. Therefore, the safety and quality of the cells must be examined carefully.
Glossary
HLA, or human leukocyte antigens, describes cell surface proteins that allow the body to distinguish self and non-self cells. HLA are found on various cell types other than leukocytes. There are tens of thousands of combinations of HLA types. HLA are classified into two types, but HLA-A, -B, and -C, which are all class 1, play a primary role in the immune response to transplanted cells.
HLA genome-edited iPS cells list
Clinical-grade cells
| HLA genome-edited iPS cells | For-profit entities | Non-profit entities |
|---|---|---|
| Clinical-grade cells | 200,000 yen/vial | Free |
- *Approval from the iPS Cell Stock Committee is required before cells can be provided.
- *Even when you apply for research purpose (non-clinical), a clinical-grade cell line is provided.Under the research condition, do not use in humans.
- *International Shipping: The receiving organization, whether profit or non-profit, is responsible for shipping and transporting arrangements and costs from CiRA Foundation to its research location. CiRA Foundation is not responsible for any loss or damage during shipment or transportation.
| Donor ID | Gene Knockout |
Clone ID |
|---|---|---|
| QHJI | ◯ | (409) QHJI14s04-AB II-KO-03 |
| QHJI | ◯ | (410) QHJI14s04-AB II-KO-11 |
| QHJI | ◯ | (411) QHJI14s04/AB II-KO-12 |
Since genome-edited clinical-grade cells can also be used for research purposes, we generally recommend clinical-grade cells for regenerative medicine. It is also possible to provide the following genome-edited research-grade cells. However, please note that research-grade cells are not the same as the above clinical-grade cells, with the two lines undergoing completely separate manufacturing processes, including genome manipulation.
Research-grade cells
| HLA genome-edited iPS cells | For-profit entities | Non-profit entities |
|---|---|---|
| Research-grade cells | 100,000 yen/vial | Free |
- *Approval from the iPS Cell Stock Committee is required before cells can be provided.
- *International Shipping: The receiving organization, whether profit or non-profit, is responsible for shipping and transporting arrangements and costs from CiRA Foundation to its research location. CiRA Foundation is not responsible for any loss or damage during shipment or transportation.
| Donor ID | Gene knockout |
Clone ID |
|---|---|---|
| DRXT | × | (401) Ff-XT28s05-cont |
| ○ | (402) Ff-XT28s05-ABo_To |
|
| QHJI | ◯ | (403) Ff-I01s04-AB II-KO-16 |
| ○ | (404) Ff-I01s04-AB II-KO-50 |
|
| ○ | (405) Ff-I01s04-AB II-KO-54 |
|
| ○ | (406) Ff-I14s04-AB II-KO-7 |
|
| ○ | (407) Ff-I14s04-AB II-KO-13 |
|
| ○ | (408) Ff-I14s04-AB II-KO-24 |
