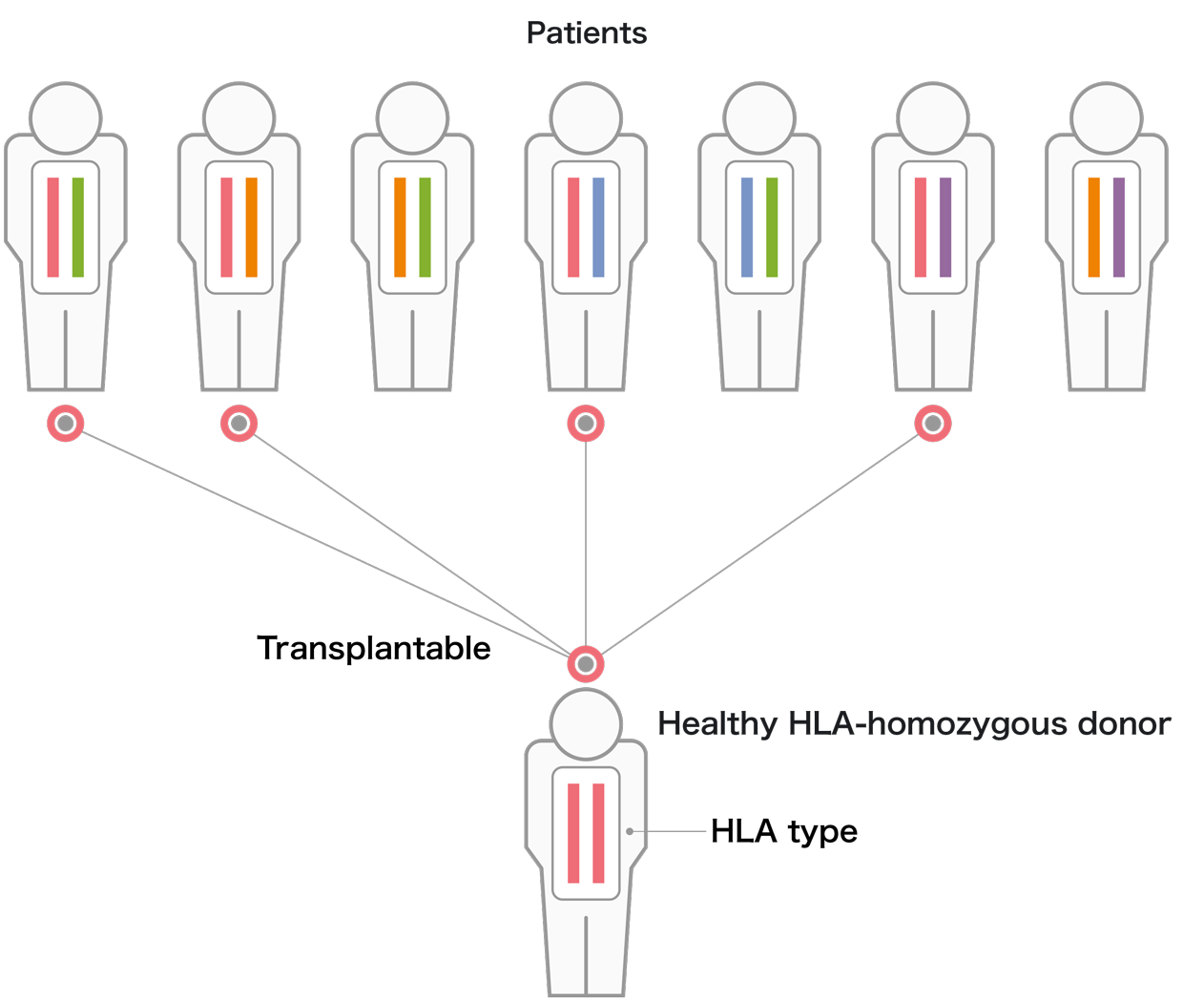
HLA homozygous iPS cells
Generated from the blood of healthy donors who are homozygous for their HLA-A, HLA-B, or HLA-DR proteins.
We manufacture and stock iPS cells with Japan's most frequent HLA types. We have thus far established a total of 27 iPS cell lines from 7 donors homozygous for 4 different HLA types, estimated to cover approximately 40% of the Japanese population. These iPS cell lines have been used in more than 10 clinical trials.
iPS cells are being made to express the most frequent HLA in Japan. Currently, a total of 27 iPS cell lines made from 7 donors who together are homozygous for 4 HLA types have been prepared.
This cell line is used in more than 10 of the clinilal trials.
Prices (excluding tax)
Research-grade cells
The research-grade iPS cells are expanded and cultured from the clinical-grade iPS cells in our laboratory.
| Service | Cost |
|---|---|
| For-profit entities | 50,000 yen/vial |
| Non-profit entities | Free |
- Because the stock of clinical-grade iPS cells is limited, researchers who have never used our iPSC Stock are offered to use the research-grade iPS cells first to check the differentiation ability.
- International Shipping: The receiving organization, whether profit or non-profit, is responsible for shipping and transporting arrangements and costs from CiRA Foundation to its research location. CiRA Foundation is not responsible for any loss or damage during shipment or transportation.
Clinical-grade cells
| Service | Cost |
|---|---|
| For-profit entities | 100,000 yen/vial |
| Non-profit entities | Free |
- We provide one vial from one clinical-grade iPS cell line with each organization in principle. If you need several vials, please contact us.
- International Shipping: The receiving organization, whether profit or non-profit, is responsible for shipping and transporting arrangements and costs from CiRA Foundation to its research location. CiRA Foundation is not responsible for any loss or damage during shipment or transportation.
Master Cell Bank derived from iPS Cell Stock
We expanded and cultured in our cell processing center(FiT) and conducted GMP-compliant QC tests. We can provide several vials even though it depends on the vial number. Please contact us for details.
| Cost |
|---|
| 200,000 yen/vial (ICH-Q5A tested) |
- International Shipping: The receiving organization, whether profit or non-profit, is responsible for shipping and transporting arrangements and costs from CiRA Foundation to its research location. CiRA Foundation is not responsible for any loss or damage during shipment or transportation.
Secondary use of iPS Cell Stock
If our iPS cell stock is to be provided to joint research partners, please be sure to contact the iPS Stock Committee, even if it is a non-profit project. Then the secondary user of our iPS Cell Stock will be charged as below.
| Service | Cost |
|---|---|
| For-profit entities | 50,000 yen/review |
| Non-profit entities | free |
- Approval from the iPS Cell Stock Committee is required before cells can be provided.
HLA homozygous iPS Cell Stock list
Overseas Regulatory
- Update on Overseas Regulatory Review
- The United States Food and Drug Administration (FDA) has informed the CiRA Foundation that the QHJI iPS cell line meets its conditions for clinical trials using iPS cell-derived dopaminergic progenitor cells in the treatment of Parkinson’s disease.
- Summary
- The CiRA Foundation has provided the QHJI iPS cell line from its iPS cell stock to Sumitomo Pharma, who has been in discussions with the FDA about a clinical trial in the United States to treat Parkinson’s disease. In this trial, dopaminergic progenitor cells made from the iPS cell line will be transplanted into patients. During the discussions, there was the realization that regulations in Japan and the United States differ regarding the requirements for using iPS cells made from the reprogramming of donor blood cells (the donor in this case is identified as QHJI). Specifically, there was concern about Title 21 CFR PRAT 1271 Subpart C – Donor Eligibility in the Code of Federal Regulations. In response, the FDA advised that QHJI be interviewed once again and another blood sample from QHJI be taken and tested.
Following the response of the CiRA Foundation, on April 29, 2022, the FDA affirmed that the iPS cells made from QHJI’s blood cells fulfill the donor eligibility requirements for the proposed clinical trials.
U.S. Drug Master File
- U.S. Drug Master File
- We have registered our iPS cell stock as a DMF to facilitate companies that are conducting research and development on regenerative medicine using our iPS cell stock to apply for approval in the U.S.
For more information, please click 「Registration of iPS cell stock (QHJI strain) for clinical use as a U.S. Drug Master File」
From peripheral blood
- * Genomic mutations in BCOR and BRD3 genes were detected in this clone.
From cord blood
HLA frequency ranking
Cooperating organization
We would like to acknowledge the following cooperating organizations for their assistance in introducing potential volunteers and providing blood collection sites for the generation of HLA homozygous iPS cells.
Potential donor introductions
- The Japanese Red Cross Society
- Japan Marrow Donor Program
- Public umbilical cord blood bank
Blood collections
- Kyoto University Hospital
