Update on the iPS Cell Stock at the CiRA Foundation
RESEARCHES
iPS Cells at the CiRA Foundation can serve 40% of the Japanese Population
Main Points
- The CiRA Foundation has published a paper in Med that provides an update on the iPS Cell Stock Project.
- The reprogramming of peripheral or cord blood from 7 donors who are homozygous for HLA-A, -B, and -DR has resulted in 27 lines of iPS cell stocks. These stocks immunologically match 40% of the Japanese population.
- The stocks have already been used in more than 10 clinical trials, with no adverse effects reported.
- The stocks have also been used by more than 60 universities and companies for research on new therapies.
- The production and quality of the iPS cells meet Good Manufacturing Practice (GMP) and other government standards.
1.Summary
To minimize the risk of immune rejection in regenerative medicines using iPS cells, iPS cell stocks using the peripheral or cord blood from donors who are homozygous for human leukocyte antigens (HLA)1 A, B, and DR have been prepared. In total, 7 donors who are homozygous for the four most frequent HLA types in Japan had their blood cells reprogrammed into 27 iPS cell stocks. These stocks are immunologically compatible with approximately 40% of the Japanese population, expanding the number of people who can benefit from iPS cell-based therapies. The distribution of the iPS Cell Stock began in 2015, and the resulting iPS cells have been used in 10 clinical trials with no adverse effects. A new study written by the CiRA Foundation summarizes the entire process, including the donor recruitment, production and quality check of the iPS cells, and their medical applications.
2.Background
The iPS Cell Stock Project for Regenerative Medicine is part of the Research Center Network for Realization of Regenerative Medicine2, which was setup by the Japan Agency for Medical Research and Development (AMED). The iPS Cell Stock Project for Regenerative Medicine originally began with the Center for iPS Cell Research and Application (CiRA), Kyoto University, but transferred to the CiRA Foundation in 2020.
iPS cells have the ability to differentiate into many cell types of the adult body, and researchers are using them to produce different tissues and organoids3 for the study of disease and treatment. For those treatments that involve transplantation, the iPS cells would preferably be made from the patient's own cells to reduce the risk of immune rejection. However, time and cost make this option unfeasible. Instead, the CiRA Foundation has been recruiting blood donors who are homozygous for HLA, since this increases the likelihood of donor-patient matching. The iPS cells produced from these donors are being shared with research organizations to quicken the development of new treatments (Fig. 1).
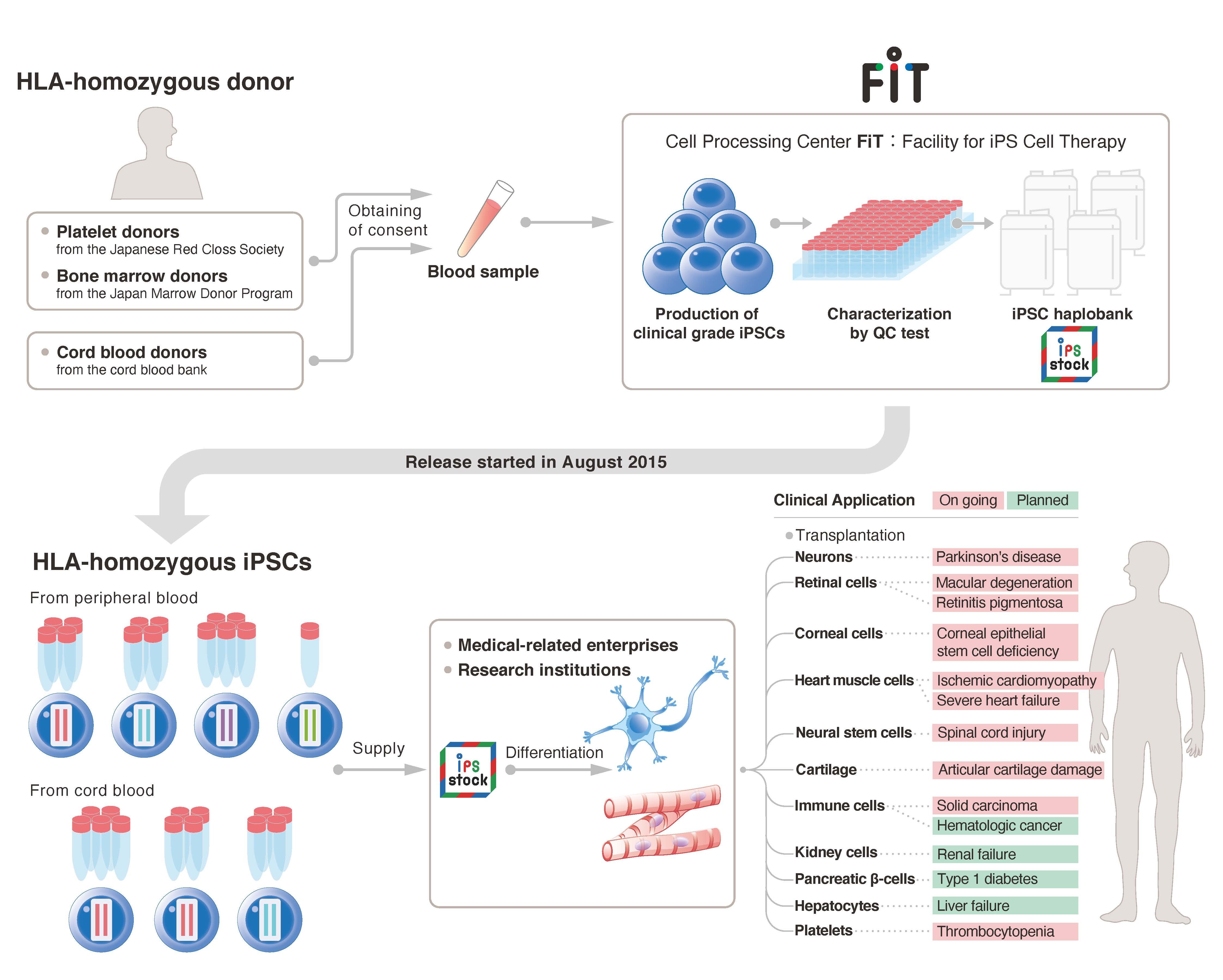
Fig 1:(cMed)
3.Main Findings
The 27 iPS cell stocks were required to undergo a number of checks before their distribution to partner research organizations. These included not only confirmation of their quality but also adhering to medical regulations for clinical use in their manufacturing and ethical regulations in the recruitment of the 7 donors. While these iPS cells stocks have already passed all quality checks, by the end of 2021 there was in total 47 donors who have qualified and are
cooperating with the CiRA Foundation. These additional donors increase the HLA-type coverage from 4 to 28 and the Japanese population coverage from 40% to 60%.
The cell production processes were divided into three different methods (Fig. 2). In the first and second methods, colony picking was done before preparing the primary cell stock, but the stock was diluted in the second method before moving on to the secondary cell stock to generate more cell lines. In the third method, no colony picking was done, which reduced the time for the production.
iPS cell reprogramming is not a perfect process, and many cells fail to pass the quality tests for several reasons. These include the presence of residual episomal vectors, which were used for the reprogramming, SNV/indels4, CNVs and structural variations5 associated with cancer-related genes, the expression of undifferentiated markers6, and karyotype abnormalities7. Upon passing these tests, the selected cells were then tested for virus and other contaminations. (Fig. 3).
The presence of episomal vectors was the main reason for Method 1 failing, but was resolved by the single cell cloning in Methods 2. As for SNV/indels, no common SNV/indels were found between the primary and secondary cell stocks, suggesting any present SNV/indels were not the result of the reprogramming. Regarding CNVs and structural variations, a common change in chromosome 11 was found, suggesting the reprogramming method still has room for improvement. The level of markers for undifferentiation was overall acceptable, but some cell stocks were excluded with this test. Finally, karyotype abnormalities were abundant in iPS cells made from one donor but not at concerning levels in iPS cells made from other donors. Karyotype abnormalities are common with age.
All iPS cells that passed shared the morphology of embryonic stem cells. Further, a gene expression analysis showed the two cell types were comparable, and any differences were more due to gender (male/female). Finally, because the medical use of iPS cells depends on their ability to differentiate, differentiation into the three germ layers was confirmed.
Notably, these iPS cells were produced following Japanese regulations, but other countries may have different criteria before the cells qualify for medical use. Earlier this year, the United States Food and Drug Administration gave approval for iPS cell stocks at the CiRA Foundation made from one donor:https://www.cira-foundation.or.jp/2022/06/30-110351.html
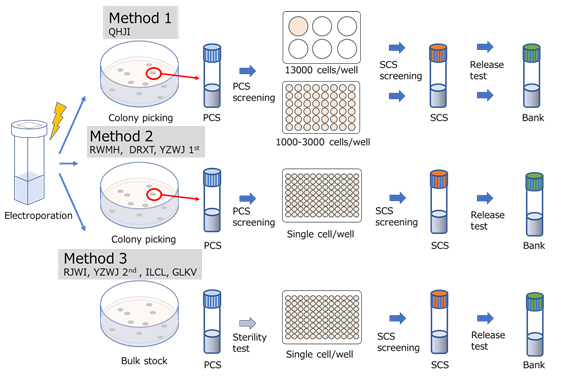
Fig 2 :(cMed)
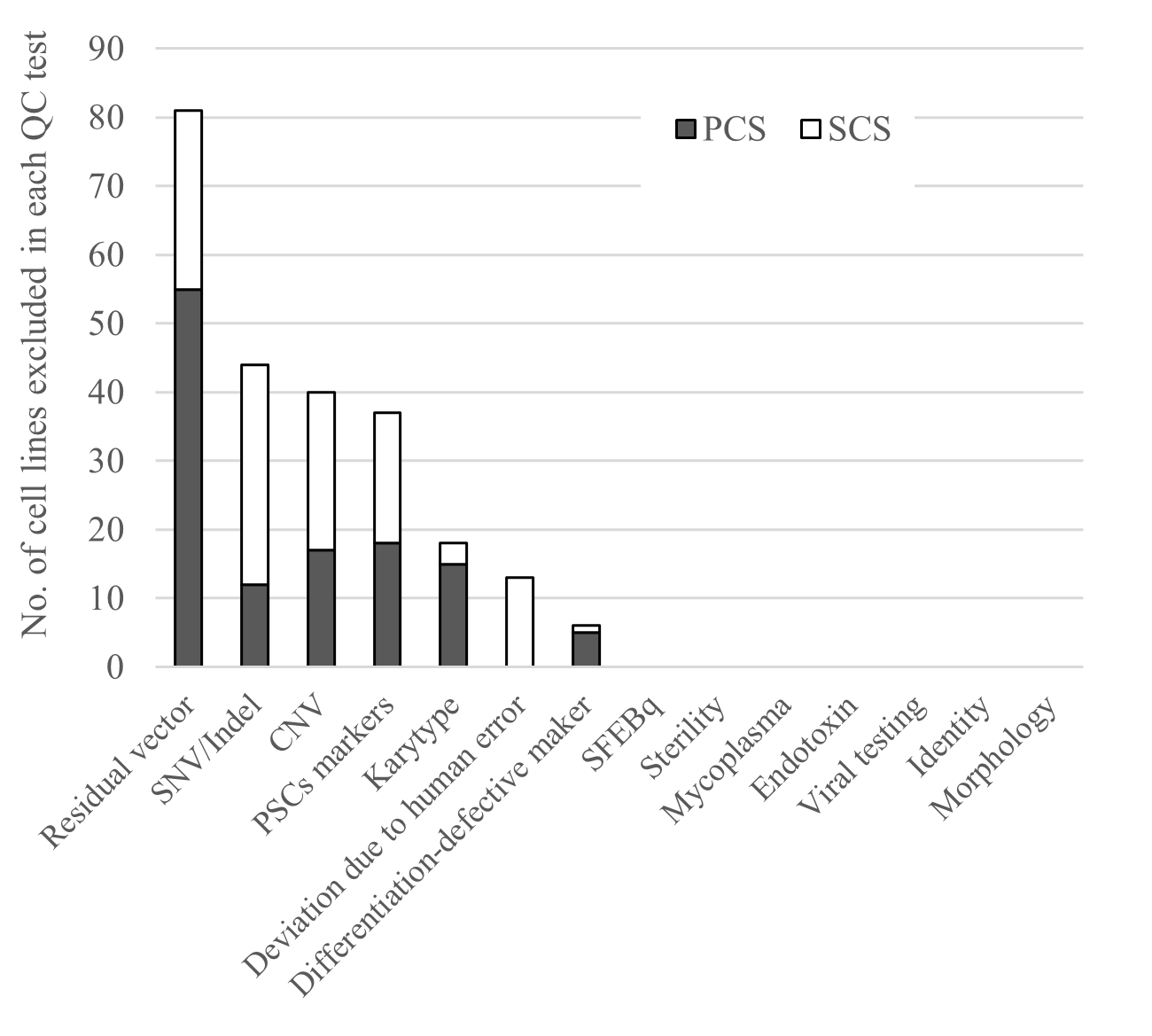
Fig 3:(cMed))
4.Future Directions
The first iPS cell stock from the CiRA Foundation was distributed in 2015, and the first clinical trial involving iPS cells began in 2017. Since then, more than 10 clinical trials8 using the CiRA Foundation iPS Cell Stock have started, and positive results have already been reported. The CiRA Foundation plans to continue sharing its stock with organizations across the world for the development of new medical treatments.
Because of the importance of HLA in donor-patient matching, the CiRA Foundation is exploring HLA genome editing so that more patients - inside and outside Japan - are eligible for iPS cell-based therapies. Additionally, further research is ongoing regarding the production and quality of iPS cells made from patient cells. This is part of the my iPS Project, which is in collaboration with several companies and universities.
5.Comment by CiRA Foundation Representative Director Dr. Shinya Yamanaka
As we begin the 10th anniversary of the iPS Cell Stock Project for Regenerative Medicine, the CiRA Foundation has published an article in the journal Med that summarizes our current accomplishments. We wish this paper to be of great use for organizations planning to enter the field.
A lot of this work has depended on many external organizations like the Japanese Red Cross Society, Japan Marrow Donor Program, and public cord blood banks, whose cooperation was critical for the recruitment of the HLA-homozygous donors. We are also greatly indebted to the many donors, research institutions, companies, and government bodies that have helped make our program a success. We want to thank everyone who supports us.
6.Details about the paper
・Title
A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population.
doi.org/10.1016/j.medj.2022.10.003
https://www.cell.com/med/fulltext/S2666-6340(22)00450-0
・Journal:
Med
・Authors:
Shinsuke Yoshida1, Tomoaki M Kato1, Yoshiko Sato2, Masafumi Umekage1, Tomoko Ichisaka1, Masayoshi Tsukahara1*, Naoko Takasu1, Shinya Yamanaka1,2,3*
*Corresponding author
・Author affiliations
1.CiRA Foundation
2.Center for iPS Cell Research and Application (CiRA), Kyoto University
3.Gladstone Institute of Cardiovascular Disease
7.Funders
Research Center Network for Realization of Regenerative Medicine, Japan Agency for Medical Research and Development (AMED)
8.Glossary
1)HLA (human leukocyte antigen)
First found in white bloods, HLA are expressed by almost all cell types in the body. HLA are used to distinguish self and foreign cells and bodies.
2)Research Center Network for Realization of Regenerative Medicine
This network was formed to make Japan a leader in the clinical application of iPS cells. It is building a system for regenerative medicine by helping establish safety and standards as well as the iPS Cell Stock.
3)Organoids
Tissues made of stem cells in a test tube that behave like specific organs
4)SNVs/indels
Single nucleotide variants describe a substituion in the gene at a single nucleotide. Insertions and deletions describe the inclusion or removal of nucleotides in the gene.
5)CNVs
Copy number variant refers to a change in the copy number of a gene in a cell.
6)Undifferentiation marker
A marker used to indicate whether a cell is in an undifferentiated state. These markers are commonly expressed by iPS cells and embryonic stem cells.
7)Karyotype aberration
A karyotype refers to the complete set of chromosomes in an individual (46 in humans; 23 from the mother and 23 from the father). An aberration suggests a change in the number or structure of the chromosomes.
8)Clinical trials
The clinical trials referred to in this article used iPS cells made by the Facility for iPS Cell Therapy (FiT) at the CiRA Foundation in partnership with other institutions.
Inquirie
For research and media inquiries
The CiRA Foundation
Public Relations Group
TEL:+81-80-2359-8495
E-mail:contact*cira-foundation.or.jp
(please change the * to @)
For AMED inquiries
Japan Agency for Medical Research and Development (AMED)
Department of Regenerative Medicine and Cell and Gene Therapies
TEL:+81-3-6870-2220
E-mail:saiseinw*amed.go.jp
(please change the * to @)
